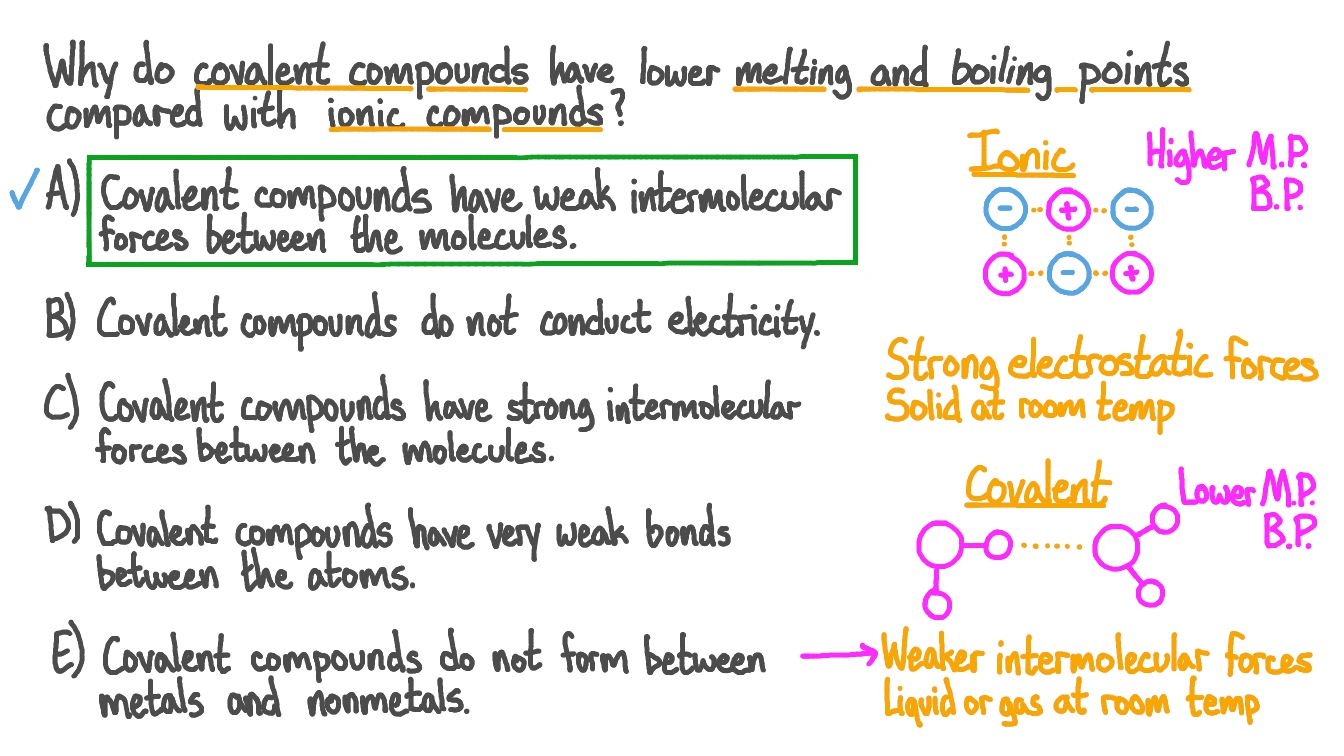
Cooking Oil Has Low Melting Point Ionic Or Covalent . comparison of properties of ionic and covalent compounds. Covalent compounds usually have lower enthalpies of. Substances can be explained by thinking about their structure and bonding. the melting and boiling points of covalent compounds are generally quite low compared to those of ionic compounds. the main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Here is an explanation of. most covalent compounds have relatively low melting points and boiling points. Because of the nature of ionic and covalent bonds, the materials produced by. differences in electronegativity classify bonds as covalent, polar covalent, or ionic.
from www.nagwa.com
Because of the nature of ionic and covalent bonds, the materials produced by. Substances can be explained by thinking about their structure and bonding. the main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Here is an explanation of. Covalent compounds usually have lower enthalpies of. differences in electronegativity classify bonds as covalent, polar covalent, or ionic. comparison of properties of ionic and covalent compounds. the melting and boiling points of covalent compounds are generally quite low compared to those of ionic compounds. most covalent compounds have relatively low melting points and boiling points.
Question Video Determining Why Covalent Compounds Have Low Melting and
Cooking Oil Has Low Melting Point Ionic Or Covalent Because of the nature of ionic and covalent bonds, the materials produced by. Covalent compounds usually have lower enthalpies of. most covalent compounds have relatively low melting points and boiling points. the melting and boiling points of covalent compounds are generally quite low compared to those of ionic compounds. differences in electronegativity classify bonds as covalent, polar covalent, or ionic. Substances can be explained by thinking about their structure and bonding. Here is an explanation of. comparison of properties of ionic and covalent compounds. the main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Because of the nature of ionic and covalent bonds, the materials produced by.
From www.slideserve.com
PPT Ionic vs. Molecular Compounds PowerPoint Presentation, free Cooking Oil Has Low Melting Point Ionic Or Covalent Substances can be explained by thinking about their structure and bonding. the main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. most covalent compounds have relatively low melting points and boiling points. differences in electronegativity classify bonds as covalent, polar covalent, or ionic. comparison of properties. Cooking Oil Has Low Melting Point Ionic Or Covalent.
From www.slideserve.com
PPT Ionic, Metallic, or Covalent? PowerPoint Presentation, free Cooking Oil Has Low Melting Point Ionic Or Covalent the melting and boiling points of covalent compounds are generally quite low compared to those of ionic compounds. differences in electronegativity classify bonds as covalent, polar covalent, or ionic. the main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Here is an explanation of. comparison of. Cooking Oil Has Low Melting Point Ionic Or Covalent.
From www.slideserve.com
PPT Heating Curve PowerPoint Presentation ID5007002 Cooking Oil Has Low Melting Point Ionic Or Covalent Here is an explanation of. Because of the nature of ionic and covalent bonds, the materials produced by. Substances can be explained by thinking about their structure and bonding. the melting and boiling points of covalent compounds are generally quite low compared to those of ionic compounds. most covalent compounds have relatively low melting points and boiling points.. Cooking Oil Has Low Melting Point Ionic Or Covalent.
From www.slideserve.com
PPT Covalent Bonding PowerPoint Presentation ID2844399 Cooking Oil Has Low Melting Point Ionic Or Covalent Covalent compounds usually have lower enthalpies of. most covalent compounds have relatively low melting points and boiling points. Substances can be explained by thinking about their structure and bonding. comparison of properties of ionic and covalent compounds. the melting and boiling points of covalent compounds are generally quite low compared to those of ionic compounds. the. Cooking Oil Has Low Melting Point Ionic Or Covalent.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID6901289 Cooking Oil Has Low Melting Point Ionic Or Covalent Substances can be explained by thinking about their structure and bonding. differences in electronegativity classify bonds as covalent, polar covalent, or ionic. the melting and boiling points of covalent compounds are generally quite low compared to those of ionic compounds. comparison of properties of ionic and covalent compounds. most covalent compounds have relatively low melting points. Cooking Oil Has Low Melting Point Ionic Or Covalent.
From slideplayer.com
Ionic and Covalent Compounds ppt download Cooking Oil Has Low Melting Point Ionic Or Covalent most covalent compounds have relatively low melting points and boiling points. Substances can be explained by thinking about their structure and bonding. differences in electronegativity classify bonds as covalent, polar covalent, or ionic. Covalent compounds usually have lower enthalpies of. the melting and boiling points of covalent compounds are generally quite low compared to those of ionic. Cooking Oil Has Low Melting Point Ionic Or Covalent.
From www.slideshare.net
Chem matters ch7_covalent_bonding Cooking Oil Has Low Melting Point Ionic Or Covalent Covalent compounds usually have lower enthalpies of. the melting and boiling points of covalent compounds are generally quite low compared to those of ionic compounds. differences in electronegativity classify bonds as covalent, polar covalent, or ionic. Here is an explanation of. Substances can be explained by thinking about their structure and bonding. the main difference between ionic. Cooking Oil Has Low Melting Point Ionic Or Covalent.
From www.numerade.com
SOLVED the comparison table below Activity 2 Comparing Cooking Oil Has Low Melting Point Ionic Or Covalent the main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Substances can be explained by thinking about their structure and bonding. differences in electronegativity classify bonds as covalent, polar covalent, or ionic. Covalent compounds usually have lower enthalpies of. comparison of properties of ionic and covalent compounds.. Cooking Oil Has Low Melting Point Ionic Or Covalent.
From jerry-has-bowman.blogspot.com
Covalent Bond Melting Point JerryhasBowman Cooking Oil Has Low Melting Point Ionic Or Covalent differences in electronegativity classify bonds as covalent, polar covalent, or ionic. the melting and boiling points of covalent compounds are generally quite low compared to those of ionic compounds. the main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Substances can be explained by thinking about their. Cooking Oil Has Low Melting Point Ionic Or Covalent.
From slideplayer.com
Covalent bonds. ppt download Cooking Oil Has Low Melting Point Ionic Or Covalent the main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. comparison of properties of ionic and covalent compounds. Here is an explanation of. the melting and boiling points of covalent compounds are generally quite low compared to those of ionic compounds. Substances can be explained by thinking. Cooking Oil Has Low Melting Point Ionic Or Covalent.
From www.chemistry-teaching-resources.com
chemistry picture Cooking Oil Has Low Melting Point Ionic Or Covalent Substances can be explained by thinking about their structure and bonding. the main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. comparison of properties of ionic and covalent compounds. Because of the nature of ionic and covalent bonds, the materials produced by. differences in electronegativity classify bonds. Cooking Oil Has Low Melting Point Ionic Or Covalent.
From www.slideserve.com
PPT Ionic, Metallic, or Covalent? PowerPoint Presentation, free Cooking Oil Has Low Melting Point Ionic Or Covalent the melting and boiling points of covalent compounds are generally quite low compared to those of ionic compounds. differences in electronegativity classify bonds as covalent, polar covalent, or ionic. most covalent compounds have relatively low melting points and boiling points. comparison of properties of ionic and covalent compounds. Because of the nature of ionic and covalent. Cooking Oil Has Low Melting Point Ionic Or Covalent.
From wagine.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps Cooking Oil Has Low Melting Point Ionic Or Covalent the melting and boiling points of covalent compounds are generally quite low compared to those of ionic compounds. Covalent compounds usually have lower enthalpies of. the main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. comparison of properties of ionic and covalent compounds. Here is an explanation. Cooking Oil Has Low Melting Point Ionic Or Covalent.
From slideplayer.com
Covalent Bonding. ppt download Cooking Oil Has Low Melting Point Ionic Or Covalent most covalent compounds have relatively low melting points and boiling points. comparison of properties of ionic and covalent compounds. Here is an explanation of. Because of the nature of ionic and covalent bonds, the materials produced by. the melting and boiling points of covalent compounds are generally quite low compared to those of ionic compounds. Substances can. Cooking Oil Has Low Melting Point Ionic Or Covalent.
From www.slideshare.net
Properties of Compounds Ionic, Covalent and Metallic Cooking Oil Has Low Melting Point Ionic Or Covalent Substances can be explained by thinking about their structure and bonding. Because of the nature of ionic and covalent bonds, the materials produced by. Here is an explanation of. differences in electronegativity classify bonds as covalent, polar covalent, or ionic. the melting and boiling points of covalent compounds are generally quite low compared to those of ionic compounds.. Cooking Oil Has Low Melting Point Ionic Or Covalent.
From themayakitchen.com
Guide to Cooking Oils by Smoke Points Online Recipe The Maya Kitchen Cooking Oil Has Low Melting Point Ionic Or Covalent Covalent compounds usually have lower enthalpies of. Here is an explanation of. most covalent compounds have relatively low melting points and boiling points. comparison of properties of ionic and covalent compounds. Substances can be explained by thinking about their structure and bonding. differences in electronegativity classify bonds as covalent, polar covalent, or ionic. Because of the nature. Cooking Oil Has Low Melting Point Ionic Or Covalent.
From slideplayer.com
Chapter 9 Covalent Bonding ppt download Cooking Oil Has Low Melting Point Ionic Or Covalent Covalent compounds usually have lower enthalpies of. comparison of properties of ionic and covalent compounds. the melting and boiling points of covalent compounds are generally quite low compared to those of ionic compounds. most covalent compounds have relatively low melting points and boiling points. Here is an explanation of. differences in electronegativity classify bonds as covalent,. Cooking Oil Has Low Melting Point Ionic Or Covalent.
From www.slideserve.com
PPT Objectives PowerPoint Presentation, free download ID5858440 Cooking Oil Has Low Melting Point Ionic Or Covalent Because of the nature of ionic and covalent bonds, the materials produced by. Covalent compounds usually have lower enthalpies of. the melting and boiling points of covalent compounds are generally quite low compared to those of ionic compounds. differences in electronegativity classify bonds as covalent, polar covalent, or ionic. Substances can be explained by thinking about their structure. Cooking Oil Has Low Melting Point Ionic Or Covalent.